Abstract
Introduction: Measurable residual disease (MRD) detected by multiparameter flow cytometry (MFC) played a key role in monitoring therapeutic efficacy and predicting prognosis for patients with acute myeloid leukemia (AML). However, identifying optimal assessing time points and incorporating MRD with other response criteria are inconclusive. Herein, based on our patient cohort data, we investigated the effect of early MRD on outcome of AML patients with different genetic-risk group and further evaluated its' impact on the allogeneic stem cell transplantation (Allo-HSCT) decision-making, which will help to establish an overall dynamic stratified prognosis system at the MRD level.
Methods: Retrospective study was conducted in patients with newly diagnosed de novo AML (acute promyeloid leukemia exclude) in three clinical trials (ChiCTR-TRC-10001202 trial, ChiCTR-TRC-10001209 trial, and NCT03021330). Patients under age 60 who achieved morphological complete remission (CR) within two courses of chemotherapy were enrolled in our study. Any detectable MRD by LAIP and/or different-from-normal were reported as MFC-MRD positive. We firstly investigated the relapse and survival of patients with different MFC-MRD status after the first course (C1) or second course (C2). Then, the outcome of incorporate MRD status in AML patients with different genetic-risk classification were evaluated. And the outcome of Allo-HSCT during the first CR and its association with the MRD status were also analyzed.
Result: The pooled analysis included 769 patients, with a median age of 38 (range 14 to 60). According to ELN 2017 recommendation, 427 (55.5%), 230 (29.9%), and 112 (14.6%) patients were classified as favorable (FR), intermediate (IR), and adverse (AR) risk groups, respectively. By evaluating relapse and survival based on MRD status, MRD positivity after C1 and C2 were both associated with significantly higher 3-year CIR (C1: 44.6% MRD+ vs. 28.3% MRD-, P < 0.001; C2 53.5% MRD+ vs. 31.0% MRD-, P < 0.001), poor RFS (47.0% vs. 66.9%, P < 0.001; 41.0% vs. 63.0%, P < 0.001) and OS (52.5% vs. 78.6%, P < 0.001; 48.0% vs. 75.6%, P < 0.001).
Hierarchical analysis revealed that after C1, MRD positivity related to significantly worse prognosis in each risk group. In FR group, CRMRD+ patients exhibited higher relapse rate compared to CRMRD- patients (3-year CIR, 34.5% vs. 23.1%, P = 0.077), as well as worse survival (3-year OS, 71.0% vs. 85.8%, P = 0.007). Similarly, in IR group, 3-year CIR was 47.2% in CRMRD+ compared to 36.0% in CRMRD- (P = 0.009), along with a worse 3-year OS (47.8% vs. 72.9%, P < 0.001). And the trend remained consistent in the AR group (CIR, 62.6% CRMRD+ vs. 37.1% CRMRD-, P = 0.026; OS, 21.2% vs. 55.5%, P = 0.002). This disadvantage became more apparent after C2, especially in FR (CIR, 40.7% CRMRD+ vs. 25.2% CRMRD-, P = 0.019; OS, 57.6% vs. 84.0%, P < 0.001) and IR (CIR, 61.5% vs. 35.2%, P < 0.001; OS, 44.2% vs. 70.7%, P < 0.001).
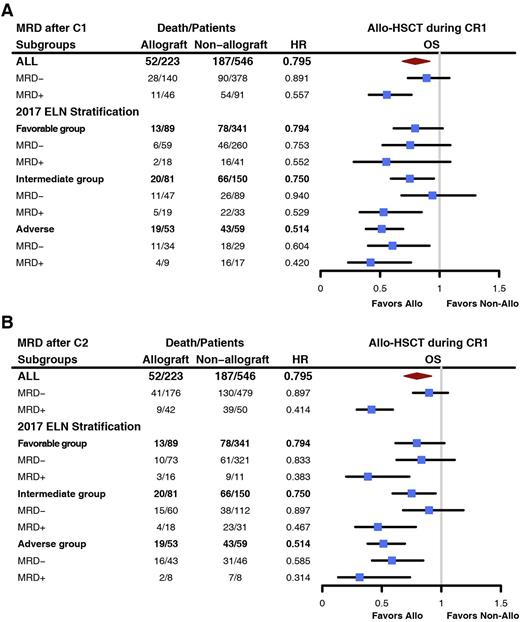
223 patients received Allo-HSCT during their CR1, 89 (39.9%), 81 (36.3%), and 53 (23.8%) patients were from FR, IR, and AR groups, respectively. Through landmark analysis and time-dependent Cox proportional hazards regression, we demonstrated that the outcome of patients with CRMRD+, particularly in FR and IR groups, was significantly improved by means of Allo-HSCT. In FR group after C2, OS of CRMRD+ patients was prolonged by Allo-HSCT during CR1 (HR, 0.383, [0.201~0.731], P = 0.004) (Figure B). In IR group, the OS of CRMRD+ patients after C1 were improved after allo-HSCT (HR, 0.529, [0.329~0.849], P = 0.008) (Figure A). Similar trend was also witnessed after C2 (HR, 0.467, [0.277~0.786], P = 0.004) (Figure B). However, OS of CRMRD- patients in FR and IR group were comparable between transplant and non-transplant patients. In AR group, regardless of MRD status, all patients could be benefited from allo-HSCT.
Conclusion: Early MRD clearance exhibited great prognostic significance, especially for patients in the favorable and intermediate risk group. Meanwhile, MRD positivity after C1 and C2 could early identify patients who need CR1 allo-HSCT in the intermediate risk group. And for patients in the favorable risk group, the evaluation time for Allo-HSCT decision-making should be after C2.
Disclosures
Wang:Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal